Select all that apply. Both of these processes can provide energy is true regarding nuclear fission and nuclear fusion.
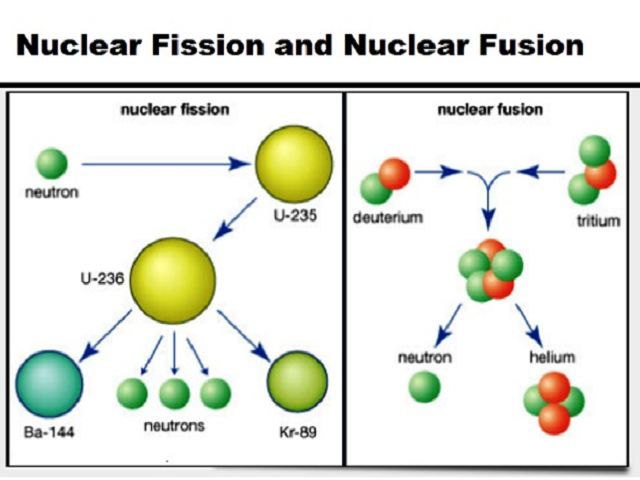
What Is The Difference Between Nuclear Fission And Nuclear Fusion
An experiment is set up in a lab to demonstrate conductivity.

. It does not occur naturally. O a fission reactor containment system D a magnetic field focused laser beams a bomb calorimeter. This causes them to split and release energy.
Rock breaking apart during a rock slide d. CRock weathers the most when first exposed then more slowly. Inside the sun Inside the earths core Inside thermonuclear weapons Inside all stars.
BThe longer the rock has been exposed the more it has weathered. Nuclear reactions can be. Both of these processes can provide energy.
The process which describes the splitting of a large unstable atom into two intermediate size atoms and extra neutrons is called nuclear fission. A crack in rock growing larger as water freezes in it b. The application of heat energy to an atomic nucleus.
The word fusion is defined as the process or result of joining two things together. Describe the process of nuclear fusion Question Which of the following may potentially be used to contain fusion reactions. A term that describes the forces that hold atomic nuclei together.
Chemistry questions and answers. Small nuclei collide and combine. Nuclear fusion is when the nuclei of two atoms fuse together forming a larger atom which releases energy.
Burrowing animals digging a home in rock c. Nuclear fusion requires more energy put in than it gives back. In this process a small amount of mass is converted into energy.
Nuclear fusion usually requires freezing temperatures. Every star uses it to produce energy. Small nuclei collide and combine.
Nuclear fusion is a nuclear reaction that combines two or more small atoms to form a large atom. Answered Jul 19 2018. Which of the following elements is the most stable from a nuclear point of view.
Which of the following are sites for nuclear fusion. In fusion the product is a larger atomic nucleus. The emission of high-energy particles or waves from atoms.
The word nuclear is derived from the word nucleus. The mass of the products is. After a few minutes the wax bead on one rod melts.
Fission produces energy but fusion does not. Nuclear fusion is a common source of energy. The proportion of hydrogen converted to energy in nuclear fusion is 7 percent.
Strong magnetic fields are used to vibrate heavy elements at an extremely rapid pace. Start studying the Quick Checks flashcards containing study terms like What is an example of chemical weathering. Only the strong nuclear force holds the nucleus together.
Which of the following statements best describes nuclear fusion. A wax bead is placed on the end of each cylindrical rod. This problem has been solved.
Which of the following best describes an exothermic nuclear fission reaction. Two light elements are given enough kinetic energy to fuse and form a heavier element while releasing energy. Nuclear fusion releases a small amount of energy compared to most chemical reactions.
Nuclear fission is a nuclear reaction that splits a heavy atom into two or more smaller ones. Decomposition of rock when. The electrons of two atoms combine to form a cluster of electrons.
The nuclear equation shows the transmutation of a form of radon into polonium and an alpha particle. Memorize flashcards and build a practice test to quiz yourself before your exam. Therefore the correct answer would be small nuclei joining together to form one larger nucleus.
Which of the following statements is true about the nucleus. Describe nuclear fusion. Which of the following statements about fusion is true.
Which of the following best describes the chain reaction that takes place during nuclear fission. See the answer See the answer See the answer done loading. The universe is full of instances of nuclear fusion reactions.
Small nuclei collide and combine to make a larger nucleus. Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles neutrons or protonsThe difference in mass between the reactants and products is manifested as either the release or the absorption of energyThis difference in mass arises due to the difference in nuclear binding energy between. The electrons of one atom separate to form electron particles.
Nuclear fission involves combining elements while nuclear fusion involves breaking down elements. Both of these processes are equally complex to control. The movement of electrons around an atomic nucleus.
Fusion produces energy but fission does not. Which describes nuclear fusion. Both of these processes can provide energy.
Fusion converts radio photons into gamma-ray photons. In fission the product is a larger atomic nucleus. In nuclear fusion smaller atoms are forced together to create larger atoms.
Our suns heat is an example of both of these processes. In one to two sentences explain whether or not the reaction is balanced. Nuclear fusion usually requires a high-temperature environment.
DTime does not affect physical weathering only. Nuclear fission involves combining elements while nuclear fusion involves breaking down elements. AOver time the rock will become flat slowing the rate of physical weathering.
Which of the following is true regarding nuclear fission and nuclear fusion. Our suns heat is an example of both of these processes. The nuclei of two atoms combine to form a larger nucleus.
The proportion of hydrogen converted to. The nucleus of one atom splits to form two smaller nuclei. Three cylindrical rods of copper iron and glass are placed in a pan of hot water with one end up.
Select all that apply.

Doe Explains Nuclear Fusion Reactions Department Of Energy

Nuclear Fusion Definition Occurrence Examples Applications Faqs
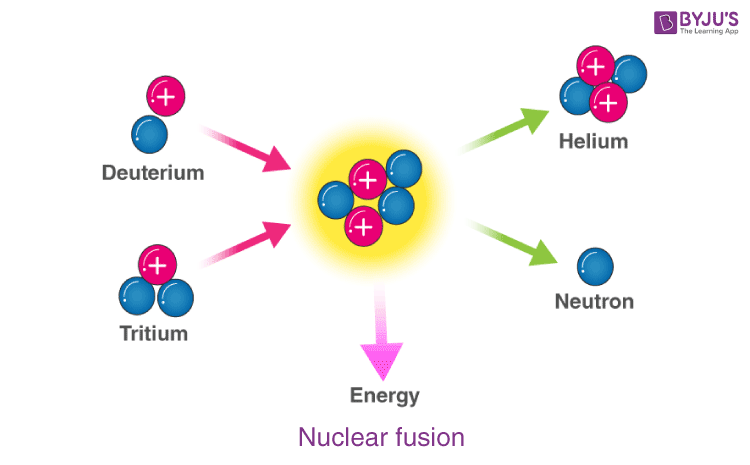
Nuclear Fusion Definition Occurrence Examples Applications Faqs
0 Comments